With a new $11.3 million Program Project grant from the National Institutes of Health, experts from the Vascular Biology Center at the Medical College of Georgia at Augusta University are working to better understand the underlying mechanisms of vascular disease.
“Blood vessels are critical for the transport of oxygen, nutrients and immune cells, and, when they don’t function properly, it not only impacts the function of the heart, but other organs, such as the lung, eye, brain and even our muscles. A key regulator of blood vessel function is a thin layer of cells lining the inside of all blood vessels, called the endothelium,” said co-program director Tohru Fukai, MD/PhD. Those endothelial cells and finding ways to fix them when they become dysfunctional is a critical focus of this new five-year grant.
Normally, endothelial cells form a tightly regulated barrier, reduce inflammation and help blood vessels to relax, allowing blood to flow. But in vascular disease, when they begin to malfunction, they can’t maintain that barrier, which leads to increased inflammation and constriction of blood vessels. That results in increased blood pressure, decreased blood flow and limited organ function.
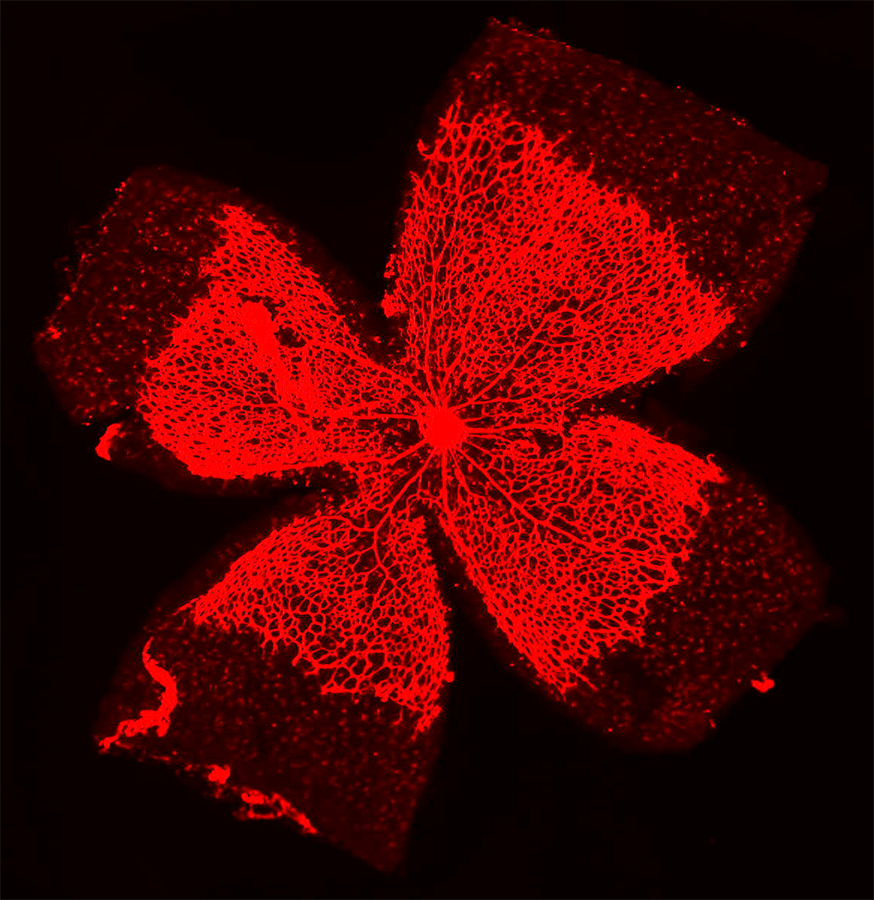
“A key problem contributing to endothelial cell dysfunction is the loss of ‘redox balance’ between harmful molecules called reactive oxygen species (ROS) and helpful molecules like nitric oxide (NO). Too much ROS and not enough NO underlies many vascular diseases,” said Masuko Ushio-Fukai, PhD, MCG vascular biologist and co-program director. “To date, treatments targeting this imbalance by reducing ROS and increasing NO haven’t been very effective. To develop more effective treatments, we need to understand what causes this imbalance.”
Scientists have recently reported that endothelial cells can reprogram the ways they create energy to meet the demands of their local environment. Normally they rely on a healthy balance between aerobic glycolysis, a metabolic process which produces energy and regulates ROS, and mitochondrial oxidation, which generates energy, ROS and various metabolites.
But in vascular disease, the balance is disrupted, and this “metabolic flexibility” often results in a shift to excessive glycolysis, which can actually shift redox balance in a negative way.

“We don’t fully understand the mechanisms regulating the relationship between endothelial metabolism and ROS/RNS balance and how it becomes altered in vascular disease,” Fukai explained. “Our program will investigate how the imbalance between endothelial metabolism and ROS/RNS that is induced by various risk factors promotes endothelial dysfunction and vascular disease.”
To make inroads into the origins of such a prevalent and complicated problem — approximately 83 million people have one or more forms of vascular disease in the United States alone — requires a collaborative approach. The MCG research team includes world-known endothelial and vascular biologists: Fukai; Ushio-Fukai; Eric Belin de Chantemele, PhD; David Fulton, PhD; David Stepp, PhD; and Rudolf Lucas, PhD.
The five-year project will include the use of shared models and resources, tightly integrated projects and the support of administrative, animal and analytic cores.

Project 1 is led by Fukai, who also serves as Barbara A. Schnuck Endowed Chair in Translational Medicine and a professor in the Department of Pharmacology and Toxicology. He will investigate how altered copper metabolism due to dysfunction of the copper exporter ATP7A leads to excessive glycolysis, redox imbalance, mitochondrial changes and, ultimately, endothelial dysfunction, which accelerates atherosclerosis.
Project 2 is led by Belin de Chantemele, professor of medicine, and Fulton, director of the VBC, who also serves as Regents Professor of Pharmacology and Toxicology. They will study how sex-specific mechanisms involving Nox1 – a key source of ROS – and the satiety hormone, leptin – a key regulator of metabolism – impact endothelial cell metabolism and function and vascular diseases, such as hypertension.
Project 3 is led by Ushio-Fukai, who is also director of the Redox Signaling Program in the VBC and a professor in the Department of Medicine. She will examine how mitochondrial dynamics coordinate crosstalk between mitochondrial redox signaling and endothelial glycolysis to regulate new blood vessel formation. Blood vessel growth will be investigated using an animal model of peripheral arterial disease with diabetes, which results in excessive glycolysis and increased ROS signaling.
These projects are also supported by three cores.
Administrative Core A is led by Fukai and Ushio-Fukai and will provide overall program support, coordinate project integration, standardized biostatistics and bioinformatics support.
Scientific Core B will provide standardized animal and metabolic phenotyping and is led by Stepp, who also serves as Leon Henri Charbonnier Endowed Chair in the Department of Physiology, Regents Professor and the medical school’s associate dean for research. Core B will support all projects by providing novel knockout or transgenic mice with altered endothelial genes that reduce and increase glycolysis, and live measurements of metabolic phenotypes.
Scientific Core C will provide endothelial cell analytics and is led by Lucas, a professor of pharmacology and toxicology, and Fulton. This core will support the isolation and characterization of primary endothelial cells from genetically modified mice and human tissue. It will also provide standardized measurements of cellular metabolism, oxidative/nitrosative stress, NO and inflammation and measuring cytokine/chemokine profiles, which are key indicators of immune function, using multiplex analysis.
This Program Project grant is also supported by trainees, technicians and administrative staff and will bring together experts from across MCG and from other universities with the common goal of reducing one of the nation’s top killers.
Discoveries at Augusta University are changing and improving the lives of people in Georgia and beyond. Your partnership and support are invaluable as we work to expand our impact.
 Augusta University
Augusta University




