Brains of veterans who had experienced explosion-caused concussions show changes in the system responsible for clearing the organ of waste and neurotoxins, a new study shows.
The finding may explain why those veterans are more likely to develop psychiatric disorders and cognitive impairment.
The paper reporting on the study appears May 28 in the journal Brain. The first author is Molly Braun, PhD, assistant professor of neurosurgery at the Medical College of Georgia at Augusta University. The paper’s senior author is Jeffrey Iliff, PhD, professor of psychiatry and behavioral sciences and neurology at the University of Washington School of Medicine, and a researcher at the VA Puget Sound Health Care System.
Traumatic brain injury due to explosions has been called the signature injury of the Iraq and Afghan wars.
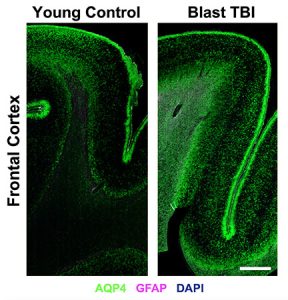
aquaporin-4 (AQP4, green) was markedly different
in the brains of veterans who sustained blast
traumatic brain injuries (right) compared to the
brains of those who had not (left). Scale bar = 2 mm.
Credit: Iliff lab
“The data suggest that nearly half of all injured service members in the Iraq war experienced neurotrauma from blast exposures,” Iliff says, “but we know little about the pathology of these injuries.”
Traumatic brain injury, due to mechanical forces such as a blow to the head, has been well studied.
“However, most studies examine impact TBI, so much less is known about brain injuries following blast exposure and how it may differ from other types of TBI,” says Braun.
In more severe injuries where the impact snaps the brain back and forth, its surface strikes the skull’s boney interior, creating tears in its soft, fragile tissue. Damage often can be clearly seen on brain scans. In mild traumatic brain injuries or concussions, the rotation of the brain during impact causes a subtler tissue injury that is not easily seen on a scan, but still causes a temporary change in, or loss of, consciousness.
Force from a blast, on the other hand, is transmitted by a pressure wave that travels through the entire brain, resulting in more diffuse tissue shearing and tearing throughout the organ.
In the new study, the researchers focused on the glymphatic system, which allows fluid to flow through the brain’s tissue during sleep, delivering nutrients and carrying away waste products.
The glymphatic system consists of cells called astrocytes, which sheathe the arteries and veins that supply the brain. This sheath creates a fluid-filled space around the blood vessels, called the perivascular space. Fluid moves between this space and the brain via pores composed of a protein called aquaporin-4 (AQP4).
It is theorized that damage to glymphatic system allows wastes to accumulate in the brain. Among the wastes removed from the brain via the glymphatic system are amyloid beta and tau proteins, which accumulate in the brains of people with Alzheimer’s disease.
A related study published in March in Neurology found that veterans with mild traumatic brain injury have abnormal levels of both amyloid and tau proteins in their cerebral spinal fluid. This finding supports the premise that such injuries impair the clearance of these proteins from the brain by the glymphatic system.
In the new study, the researchers compared brain tissue from veterans who had sustained a blast-related traumatic brain injury with those who had not. The researchers found that the distribution of protein AQP4 was markedly abnormal in veterans with these injuries, compared to those without. These abnormalities were concentrated in areas where blast-wave forces are thought to be most pronounced, just below the brain’s surface at the interface between gray and white matter.
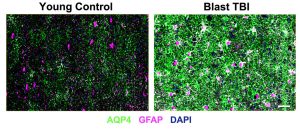
expression of aquaporin-4 (AQP4, green) and
glial fibrillary acidic protein (GFAP, magenta)
were markedly different in the brains of veterans
who sustained blast traumatic brain injuries (right)
compared to the brains of those who had not (left).
Scale bar = 50 µm. Credit: Iliff lab
“The AQP4 changes we saw in this human brain tissue were quite striking. Typically, we focus on changes in AQP4 down at a more cellular level but, in these blast-exposed veterans, we saw prominent AQP4 changes across entire regions of the brain tissue,” says Braun.
To see if these changes could impair the glymphatic system, the investigators studied the distribution of AQP4 in mice that had been exposed to a series of blast waves mimicking those experienced by servicemembers. The researchers found a similar abnormal pattern.
In these animals, the changes in AQP4 were accompanied by a marked slowing in the brain’s waste-clearance system, a change that demonstrated that blast traumatic brain injury impairs this process.
Finally, the researchers compared MRI brain scans of veterans with a history of blast traumatic brain injuries with those who did not. They found an increase in the number of enlarged perivascular spaces, which is thought to be a sign of impaired glymphatic function.
“By combining studies in human postmortem brain tissue, from rodent models of blast TBI, and from human brain imaging, we get a clearer view of the relationship between blast TBI and glymphatic function than we could get from human studies or rodent studies alone,” Iliff says.
“We see that people who experienced blast TBI exhibit clear changes in their brains that, in rodents, impair glymphatic function,” he added. “The MRI findings confirm that veterans with blast TBI, especially those with poor sleep, appear to have evidence for impairment of this process.”
Iliff concluded, “These findings suggest that changes in AQP4 and glymphatic impairment following blast injury may leave the brain vulnerable to post-concussive symptoms like depression and post-traumatic stress disorder, and may over the long term lead to dementing disorders like Alzheimer’s disease or chronic traumatic encephalopathy.”
“The hope is that these studies will help lay the groundwork for the development of future treatments or even equipment for preventing these blast TBIs,” Braun says. “Once we have a better understanding of the injury and how it progresses over time, through studies like this, we can start targetting these processes therapeutically.”
This work was supported by the National Heart, Lung, and Blood Institute (K23HL150212-01), Department of Veterans Affairs Rehabilitation Research and Development Service Merit Review (B77421), Department of Veterans Affairs Biomedical Laboratory Research and Development Service Merit Review (BX005735) National Institute of Aging (AG066518), and the Oregon Alzheimer’s Research Center (P30AG066518).
Media Contact: UW Medicine: Barbara Clements, bac60@uw.edu
 Augusta University
Augusta University




