An Augusta University researcher is partnering with the University of California San Diego to unravel the complexities of Staphylococcus aureus vaccine failures.
Wan Tien “Austin” Chiang, PhD, assistant professor with the Immunology Center of Georgia (IMMCG), will take part in a $3.3 million, five-year grant from the National Institutes of Health to support the project, “Mechanisms of vaccine interference by S. aureus-induced imprints.”
Staphylococcus aureus is a globally prevalent pathogen and a major contributor to antibiotic resistance. Despite numerous attempts, previous vaccine trials have faced setbacks, leaving researchers puzzled about the reasons behind these failures. Chiang’s research will use a combination of systems immunology and “big data” to investigate what is behind the inefficacy of vaccines against this pathogen.
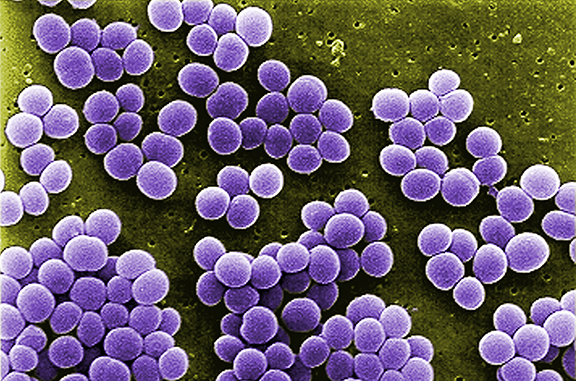
“Although there have been close to 30 vaccine trials, all successful pre-clinical vaccines taken to human trials have failed for unclear reasons,” Chiang said. “The goal of this study is to mechanistically investigate the reasons for the failure of vaccines against this pathogen, which is a leading cause of infection worldwide. Our focus is on exploring the role of S. aureus-induced immune imprints and their impacts on the immune response.”
Central to Chiang’s approach is the integration of systems immunology, an interdisciplinary field combining immunology with systems biology, computational biology and bioinformatics. By leveraging advanced single-cell multi-omics technologies, the research team aims to comprehensively understand the immune system’s complexities underlying the intricate interactions between S. aureus, the host, and the vaccines.
“The findings of this research could pave the way for safer and more effective vaccines, with practical applications in addressing global health challenges. This project is a great example of the power of interdisciplinary approaches in unlocking the secrets of the immune system.”
Wan Tien “Austin” Chiang, PhD, IMMCG assistant professor
In collaboration with George Y. Liu, MD, PhD, principal investigator at the UCSD School of Medicine, and Klaus Ley, MD, founding co-director of IMMCG at the Medical College of Georgia, Chiang aims to address the longstanding challenge of developing a successful vaccine.
“If the findings from this research are successful, they could have profound implications for both vaccine development and immunotherapy across a broad range of applications,” said Ley. “Understanding the mechanisms behind the immune system’s response to vaccines could lead to the design of more effective vaccines against various pathogens.”
The utilization of big data, particularly through single-cell RNA sequencing, provides crucial insights into the mechanisms underlying immune responses. Chiang notes the importance of this approach to identify the specifics involved in the immune response to S. aureus, such as the role of glycans, which serve numerous purposes in the protection, stabilization, organization and barrier functions of cells.
“We employ single-cell RNA sequencing analyses to identify responsible glycosylation enzymes and specific glycan modifications, cellular receptors, and mechanisms in B-cell and T-cell immunity,” Chiang said. “This approach allows us to explore the intricacies of the immune system comprehensively and holistically.”
Recognizing the complexity of the research, Chiang emphasizes the importance of interdisciplinary collaborations. Bioinformaticians, immunologists and glycobiologists are all crucial contributors, each providing unique expertise that collectively contributes to breakthroughs in understanding and manipulating the immune system for vaccine development.
Chiang envisions a future where predictive capabilities enhance vaccine development, leading to more precise, personalized and efficient vaccines.
“The findings of this research could pave the way for safer and more effective vaccines, with practical applications in addressing global health challenges,” Chiang said. “This project is a great example of the power of interdisciplinary approaches in unlocking the secrets of the immune system.”
 Augusta University
Augusta University